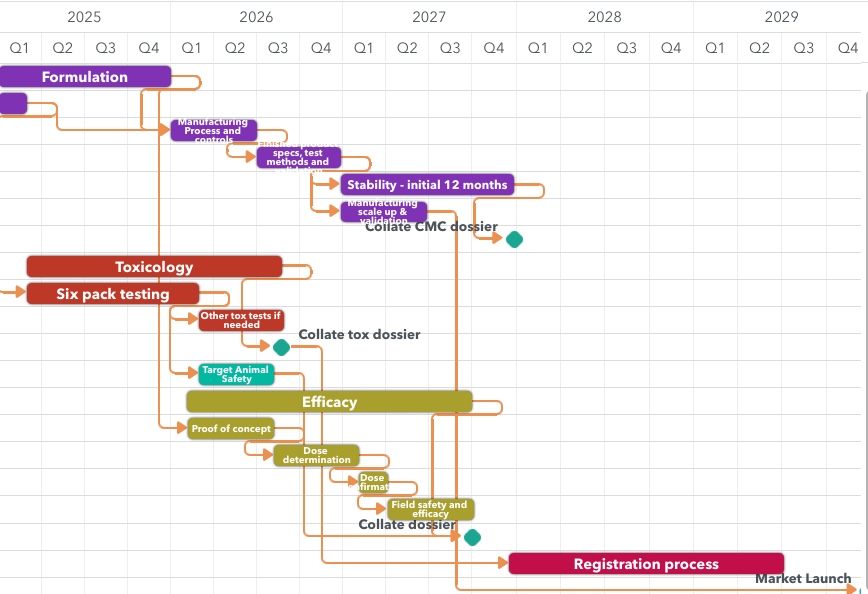
Bring products to market faster with scientifically robust, GLP/GCP-compliant trials across New Zealand and Australia. Our expertise spans every step — from protocol design to regulatory-ready results.

Our team of veterinary, epidemiology , and regulatory professionals span across New Zealand and Australia and make us one of the largest and full-service GLP-accredited Contract Research Organisations in Australasia.
Our expertise spans every step, from protocol design to regulatory-ready results. We understand the nuances of navigating the path you are on, and can partner with you at every step.






From pioneering new treatments to revolutionising diagnostic tools, our clients’ successes are a reflection of our shared ambition to push boundaries and set new standards in animal health care.
Dive into the case studies of products we have worked on that have not only thrived in the competitive markets of Australia and New Zealand but have also made a tangible difference in the lives of animals and their caregivers.
From pioneering new treatments to revolutionising diagnostic tools, our clients’ successes are a reflection of our shared ambition to push boundaries and set new standards in animal health care.

One partner from design through to registration
Independently audited for quality and compliance
Seamless process, high standards of welfare and compliance
National farm networks for multi-site, real-world data
Data-driven decisions, defensible results
Whether you’re seeking guidance on the next steps, looking for answers to specific questions, or eager to discuss how our services can support your product’s journey, we’re here to help.